Modeling and design of many types of equipment for separating gas and liquids such as flash separators at the well head, distillation columns and even a pipeline are based on the phases present being in vapor-liquid equilibrium. The thermodynamic equilibrium between vapor and liquid phases is expressed in terms equality of fugacity of component i in the vapor phase, fiV, and the fugacity of component i in the liquid phase, fiL, is written as
- De Priester Charts Calculator Online
- De Priester Charts Calculator online, free
- Depriester Chart Calculator Online
- De Priester Charts Calculator Online Calculator
Equation (1) is the foundation of vapor-liquid equilibrium calculations; however, we rarely use it in this form for practical applications. For calculation purposes, Eq. (1) is transformed to a more common expression which is
Ki is called the vapor–liquid equilibrium ratio, or simply the K-value, and represents the ratio of the mole fraction in the vapor, yi, to the mole fraction in the liquid, xi. Equation (2) is also called “Henry’s law” and K is referred to as Henry’s constant. For the more volatile components the Kvalues are greater than 1.0, whereas for the less volatile components they are less than 1.0.
Depending on the system under study, any one of several approaches may be used to determine K-values. Obviously, experimental measurement is the most desirable; however, it is expensive and time consuming. Alternatively, there are several graphical or numerical tools that are used for determination of K-values. This “Tip of the Month” presents a history of many of those graphical methods and numerical techniques.
In general K-values are function of the pressure, temperature, and composition of the vapor and liquid phases. The components making up the system plus temperature, pressure, composition, and degree of polarity affect the accuracy and applicability, and hence the selection, of an approach. The widely used approaches are K-value charts, Raoult’s law, the equation of state (EoS) approach (f), activity coefficient approach (?) or combination of EoS and the EoS and ? approaches [1-5]. EoS approach requires use of a digital computer.
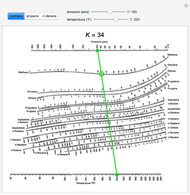
DePriester Charts present an efficient method in finding the equilibirum ratios for different substances at different conditions of pressure and temperature. These nomograms have a co-ordinate each of pressure and temperature and have 'K' values ingeniously plotted in between. To see how to use the DePriester Charts, right.
K-Value Charts
There are several forms of K-value charts. One of the earliest K-value charts for light hydrocarbons is presented in reference [1]. In these charts, K-values for individual components are plotted as a function of temperature on the x-axis with pressure as a parameter. In each chart the pressure range is from 70 to 7000 kPa (10 to 1000 psia) and the temperature range is from 5 to 260 ºC (40 to 500 ºF).
Early high pressure experimental work revealed that, if a hydrocarbon system of fixed overall composition were held at constant temperature and the pressure varied, the K-values of all components converged toward a common value of unity (1.0) at some high pressure. This pressure was termed the “Convergence Pressure” of the system and has been used to correlate the effect of composition on K-values, thus permitting generalized K-values to be presented in a moderate number of charts.
DePriester Charts provide an efficient method to find the vapor-liquid equilibrium ratios for different substances at different conditions of pressure and temperature. The original chart was put forth by C.L. DePriester in an article in Chemical Engineering Progress in 1953. These nomograms have two vertical coordinates, one for pressure, and another for temperature. 'K' values, representing. The ALCON ® Online Toric IOL Calculator With the barrett toric algorithm Theoretically accounts for posterior corneal astigmatism Most accurate preoperative prediction of residual astigmatism 1-3. Extended to the more general determination of the de-grees of freedom (number of allowable speci–cations) for a ⁄ow system, including consideration of extensive vari-ables. The intensive and extensive variables are related by material and energy balance equations together with phase equilibrium data in the form of equations, tables,. Area Calculator. This planimeter tool can be used to measure the enclosed area of a defined polyline on a map. 11th July 2018 Unfortunately, due to a large price increase in back-end services, we can no longer offer some features on this page.
In more recent publications [2], the K-values are plotted as a function of pressure on the x-axis with temperature and Convergence Pressure as parameters. In order to use these charts, one should determine the Convergence Pressure first. The determination of convergence Pressure is a trial-and-error procedure and can be found elsewhere [6].
For computer use, later in 1958 these K-Value charts were curve fitted to the following equations by academic and industrial experts collaborating through the Natural Gas Association of America [7].
In Eq (3) T is temperature in ºR, P is pressure in psia and the fitted values of the bij coefficients are reported in an NGAA publication [7].
A relatively simple nomograph is normally presented in undergraduate thermodynamics and unit operations text books. In the nomograph, the K-values of light hydrocarbons, normally methane through n-decane, are plotted on one or two pages. Charts of this type do allow for an average effect of composition, but the essential basis is Raoult’s law and equilibrium constants derived from them are useful only for teaching and academic purposes.
Raoult’s Law
Raoult’s Law is based on the assumptions that the vapor phase behaves as an ideal gas and the liquid phase is an ideal solution. Under these conditions the fugacities are expressed as
The saturation pressure of a component is represented by PiSat and the pressure of the system is represented by P. Substituting from Eqs (4) and (5) in Eq (1) gives
The vapor pressure may be read from a Cox chart or calculated from a suitable equation in terms of temperature. A typical Cox chart may be found in reference [8]. The Antoine [5] equation is recommended for calculating vapor pressure:
Values of A, B, and C for several compounds are reported in the literature [5]. Complex vapor pressure equations such as presented by Wagner [5], even though more accurate, should be avoided because they can not be used to extrapolate to temperatures beyond the critical temperature of each component. Raoult’s law is applicable to low pressure systems (up to about 50 psia or 0.35 MPa) or to systems whose components are very similar such as benzene and toluene. This method is simple but it suffers when the temperature of the system is above the critical temperature of one or more of the components in the mixture. At temperatures above the critical point of a component, one must extrapolate the vapor pressure which frequently results in erroneous K-values. In addition, this method ignores the fact that the K-values are composition dependent.
Correlation Method
As mentioned earlier, determination of K-values from charts is inconvenient for computer calculations. Therefore, scientists and engineers have developed numerous curve fitted expressions for calculation of K-values. However, these correlations have limited application because they are specific to a certain system or applicable over a limited range of conditions. Some of these are polynomial or exponential equations in which K-values are expressed in terms of pressure and temperature. One of these correlations presented by Wilson [9], is:
where Tci, critical temperature, in ºR or K, Pci, critical pressure, in psi, kPa or bar, ?i is the acentric factor, P is the system pressure, in psi, kPa or bar, T is the system temperature, in ºR or K. (P and Pc, T and Tc must be in the same units.) This correlation is applicable to low and moderate pressure, up to about 3.5 MPa (500 psia), and the K-values are assumed to be independent of composition.
EoS Approach
The fugacity of each component is determined by an EoS. In other words, both phases are described by only one EoS. It is a powerful tool and relatively accurate if used appropriately. This approach is widely used in industry for light hydrocarbon and non polar systems. Under these conditions the fugacities are expressed by
The fugacity coefficients for each component in the vapor and liquid phases are represented by ?iV and ?iL
, respectively. Substitution of fugacities from Eqs (9) and (10) in Eq (1) gives
De Priester Charts Calculator Online
The EoS method has been programmed in the GCAP for Volumes 1 & 2 of Gas Conditioning and Processing Software to generate K-values using the SRK EoS [10].
EoS-Activity Coefficient Approach
The approach is based on an EoS which describes the vapor phase non-ideality through the fugacity coefficient and an activity coefficient model which accounts for the non-ideality of the liquid phase. This approach is widely used in industry for polar systems exhibiting highly non-ideal behavior. Under these conditions the fugacities are expressed by
The fugacity coefficients for each component in the vapor phase are represented by fiV . The saturation fugacity coefficient for a component in the system, fiSat is calculated for pure component i at the temperature of the system but at the saturation pressure of that component. Normally, an EoS is used to calculate both fiV and fiSat . Substitution of fugacities from Eqs (12) and (13) in Eq (1) gives
Activity coefficients are calculated by an activity coefficient model such as that of Wilson [11] or the NRTL (Non-Random Two Liquid) model [12]. In order to calculate K-values by equation 14, the mole fractions in both phases in addition to the pressure and temperature must be known. Normally not all of these variables are known. As is the case for the EoS approach, calculations are trial and error. This approach is applicable to polar systems such as water – ethanol mixtures from low to high pressures.
De Priester Charts Calculator online, free
Normally, for low pressures, we can assume that the vapor phase behaves like an ideal gas; therefore both ?iV and ?iSat are set equal to 1.0. Under such circumstances, Eq (14) is reduced to
Eq (15) is applicable for low pressure non-ideal and polar systems. Assuming the liquid phase is an ideal solution, ? i becomes unity and Eq (15) is reduced further to a simple Raoult’s law.
Depriester Chart Calculator Online
The JMC K-Values
Two sets of K-values are summarized in Appendices 5A and 5B at the end of Chapter 5 of Gas Conditioning and Processing, Vol. 1. Appendix 5A is a series of computer-generated charts using SRK EoS. The values shown are useful particularly for calculations of vapor liquid equilibrium wherein liquid being condensed from gas systems. Appendix 5B is based on the data obtained from field tests and correlations on oil-gas separators. The data set was based on over 300 values. This correlation has bee used for often for oil separation calculations.
To learn more on applications of K-values and their impact on facilities calculation, design and surveillance, refer to JMC books [12-13] and enroll in our G4 (Gas Conditioning and Processing) and G5 (Gas Conditioning and Processing – Special) courses.
By Dr. Mahmood Moshfeghian
Reference:

De Priester Charts Calculator Online Calculator
- Natural Gasoline Supply Men’s Association, 20th Annual Convention, April 23-25, 1941.
- Engineering Data Book, 10th and 11th Editions, Gas Processors and Suppliers Association Data Book, Tulsa, Oklahoma, (1998).
- Prausnitz, J. M.; R. N. Lichtenthaler, E. G. de Azevedo, “Molecular Thermodynamics of Fluid Phase Equilibria,”, 3rd Ed., Prentice Hall PTR, New Jersey, NY, 1999.
- Maddox, R. N. and L. L. Lilly, “Gas conditioning and processing, Volume 3: Advanced Techniques and Applications,” John M. Campbell and Company, Norman, Oklahoma, USA, 1994.
- Reid, R. C.; J. M. Prausnitz, and B. E. Poling, “The properties of Gases and liquids,” 4th Ed., McGraw Hill, New York, 1987.
- Engineering Data Book, 7th Edition, Natural Gas Processors Suppliers Association, Tulsa, Oklahoma, 1957.
- Equilibrium Ratio Data for Computers, Natural Gasoline Association of America, Tulsa, Oklahoma, (1958).
- Natural Gasoline and the Volatile Hydrocarbons, Natural Gasoline Association of America, Tulsa, Oklahoma, (1948).
- Wilson, G., “A modified Redlich-Kwong equation of state applicable to general physical data calculations,” Paper No15C, 65th AIChE National meeting, May, (1968).
- G. Soave, Chem. Eng. Sci. 27, 1197-1203, 1972.
- Wilson, G. M., J. Am. Chem. Soc. Vol 86, pp.127-120, 1964
- Campbell, J. M. “Gas conditioning and processing, Volume 1: Fundamentals,” John M. Campbell and Company, Norman, Oklahoma, USA, 2001.
- Campbell, J. M., “Gas conditioning and processing, Volume 2: Equipment Modules,” John M. Campbell and Company, Norman, Oklahoma, USA, 2001.